Partners and companies within ECCPM develop implementable solutions to propel pharmaceutical manufacturing into the twentyfirst century.
BETTER MANUFACTURING NEEDS BIGGER THINKING
The Drivers for Modernizing Pharmaceutical Manufacturing
- Process and material understanding
- QA / QC strategy
- Real-time release testing
- Predictive modeling
- Regulatory alignment
- Economic alignment
- Human resource/expertise development
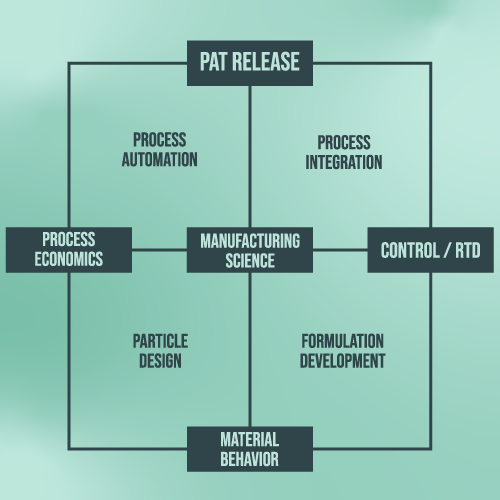
Our Approach to Continuous Manufacturing Development:
- An interdisciplinary team of process, pharmaceutical and chemical engineers and researchers
- A diverse partner matrix consisting of pharma, technology/equipment providers, and academic/scientific collaborators
- A platform for pre-competitive information exchange and networking
- Flexible intellectual property rights (IPR) arrangements to enable a agile and focused exploration of specific interest
Benefits and Expected Outcomes
- Low financial risks to explore new technological options and applications
- Access to RCPE’s long-term experience with continuous and advanced manufacturing, PAT, process control, and predictive modeling
- Exploitation of RCPE’s extensive experience in the advanced manufacturing and (in-line and off-line) characterization
- Access to the most advanced AI-based dissolution prediction models
- Access to the regular ECCPM-organized conferences
- Examination of the impact of continuous manufacturing routes on costs (production, materials, footprint, etc.), efficiency and yield
- Implementation of concepts for integrated quality control strategies
- Utilization of continuous manufacturing knowledge and experience
- Access to consolidated scientific expertise (e.g., simulation, control and process knowhow, RTRT-PAT, and formulation development)
- Access to equipment and instrumentation suppliers
- Access to an international and focused network




